
CE-IVD marked AI tool that detects and grades prostate adenocarcinoma in core needle biopsy specimens
Vendor
Indica Labs
Company Website
AI-Powered Diagnostic
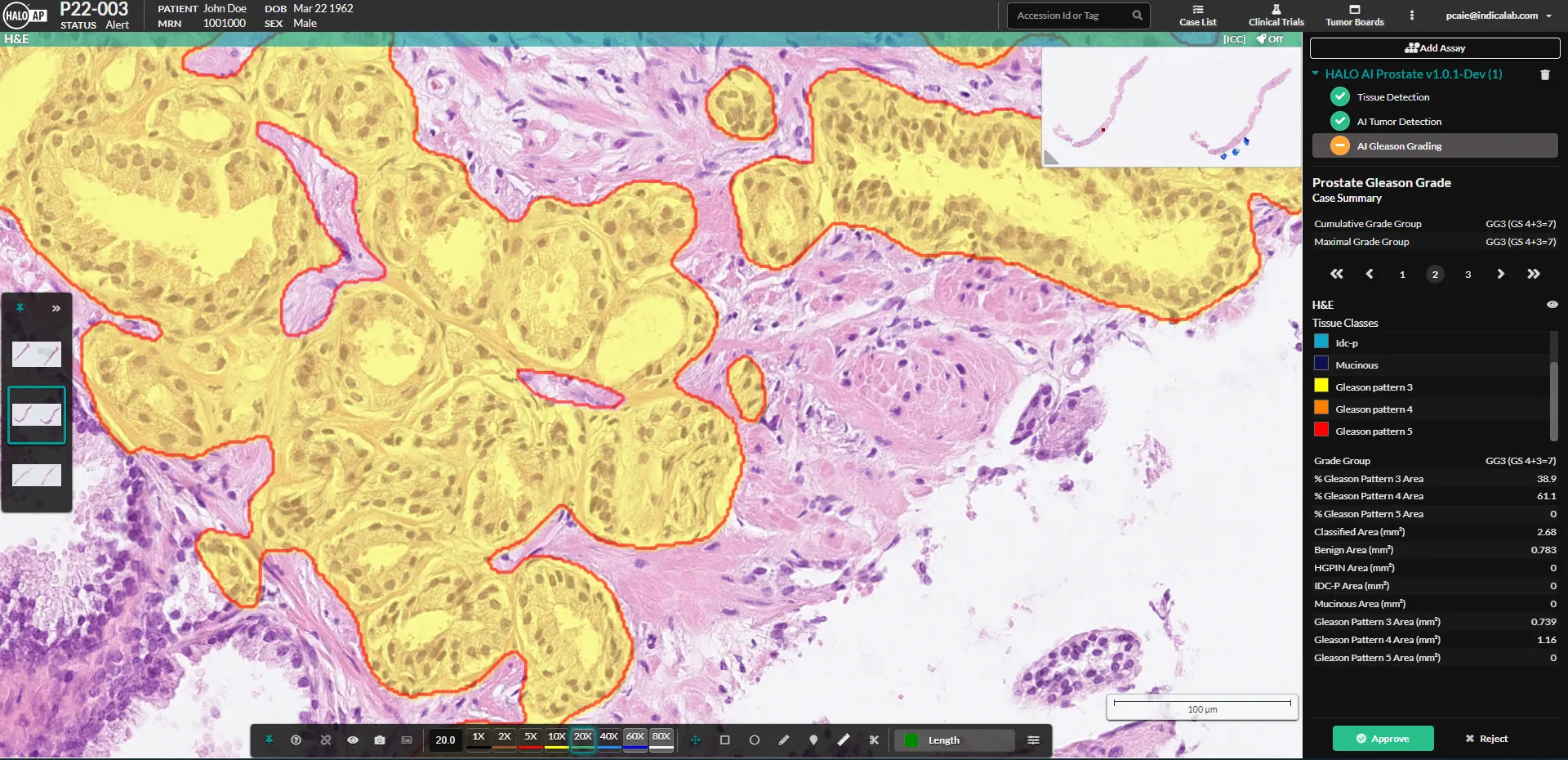
HALO Prostate AI, from Indica Labs, is a CE-IVD certified deep learning based screening tool designed to assist pathologists in the identification and grading of prostate cancer in core needle biopsies. The algorithm automatically analyzes all appropriate case slides and notifies pathologists of cases with suspected findings directly in their native workflow.
Comprehensive Analysis
The algorithm reports a comprehensive set of results and markups, including cancer detection and localization, tumor size, Gleason grading, the presence of high-grade PIN and intraductal carcinoma.
Seamlessly Integrated, AI-Enhanced Workflow
- CE-IVD marked with best-in-class clinical sensitivity and specificity
- Deploy as a screening tool to support triage or as a post-diagnostic QC-check
- Results can be shared with LIS | LIMS systems from any vendor
- Deploys in your own IT environment – cloud or on-premise – so you maintain full control of the data
- Extend your image analysis capability with further integration of IHC image analysis from Indica Labs
File Formats
- Non-proprietary (JPG, TIF, OME.TIFF)
- Nikon (ND2)
- 3DHistech (MRXS)
- Akoya (QPTIFF, component TIFF)
- Olympus / Evident (VSI)
- Hamamatsu (NDPI, NDPIS)
- Aperio (SVS, AFI)
- Zeiss (CZI)
- Leica (SCN, LIF)
- Ventana (BIF)
- Philips (iSyntax, i2Syntax)
- KFBIO (KFB, KFBF)
- DICOM (DCM*)
- *whole-slide images
Seamless Deployment in HALO AP®
HALO Prostate AI is deployed and fully integrated into HALO AP®, the AI-powered, pathologist-driven platform for anatomic pathology workflows from Indica Labs.
Regulatory Compliance
HALO AI Prostate is CE-marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AI Prostate is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. HALO AI Prostate is accessed via the HALO AP® enterprise digital pathology platform. HALO AP® is CE-IVDR marked for in-vitro diagnostic use in Europe, the UK, and Switzerland. HALO AP is For Research Use Only in the USA and is not FDA cleared for clinical diagnostic use. In addition, HALO AP provides built-in compliance with FDA 21 CFR Part 11, HIPAA, and GDPR.