
Cloudbyz Clinical Trial Management System (CTMS) is a cloud-based solution built on Salesforce that enables end-to-end management of clinical trials with real-time visibility, centralized data, and streamlined collaboration across study planning, budgeting, execution, and close-out.
Vendor
Cloudbyz
Company Website
Clinical Trial Management System (CTMS)
Cloudbyz Clinical Trial Management System (CTMS) is a comprehensive, cloud-based solution built on the Salesforce platform, designed to manage and streamline clinical trial operations across all phases. It provides real-time visibility, centralized data access, and collaborative tools for sponsors, CROs, and research sites. The system supports planning, budgeting, site management, subject tracking, monitoring, and trial close-out, enabling faster, more efficient, and compliant clinical research execution.
Features
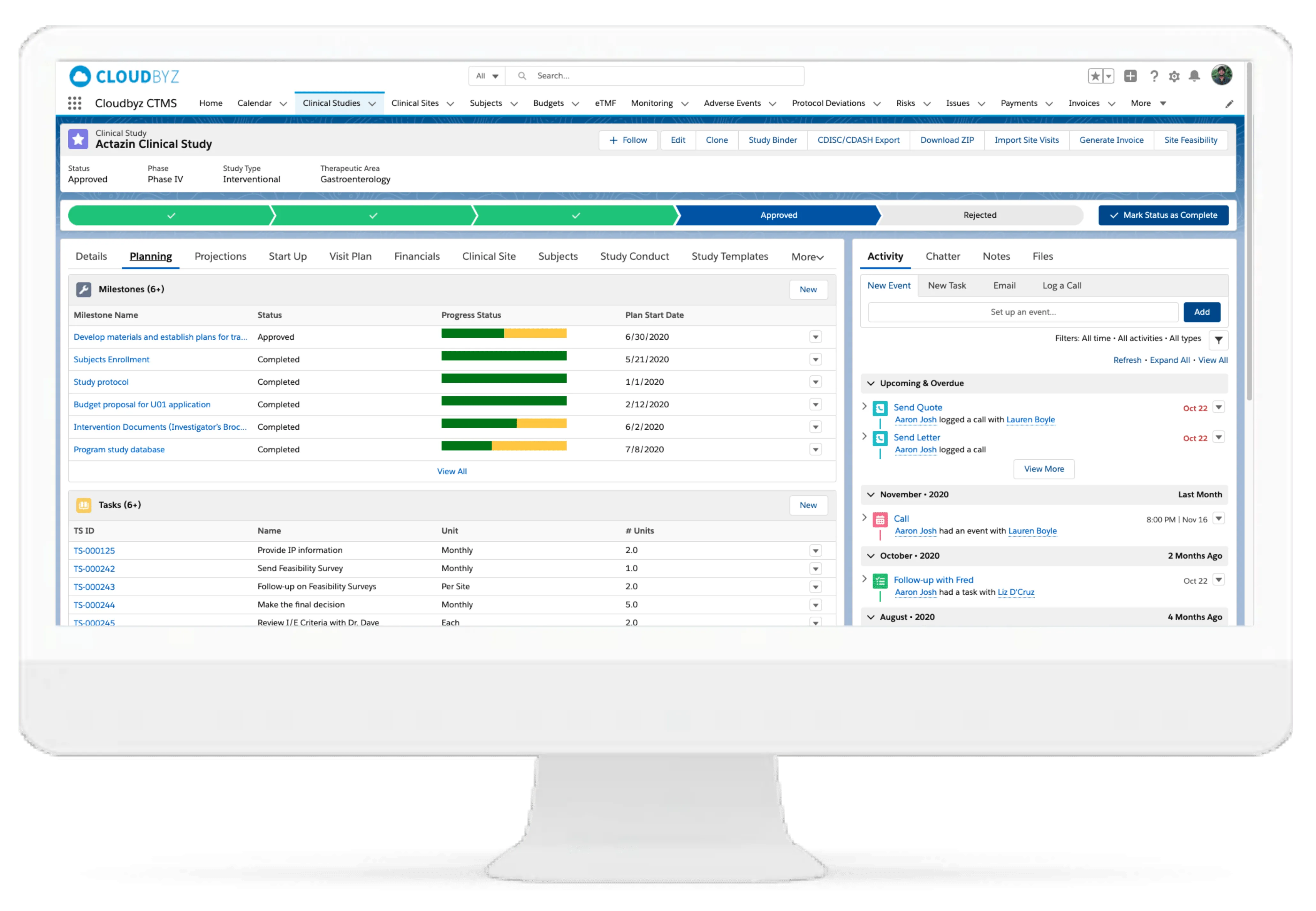
- Project and Study Management: Set up and manage studies with detailed protocol tracking, milestones, tasks, and team assignments.
- Site Management: Assign and monitor sites, manage documentation, credentialing, equipment, and activation status.
- Site Feasibility: Create and customize feasibility templates, send surveys, and evaluate responses with dashboards.
- Subject Management: Track subject visits, procedures, and payment policies in real time.
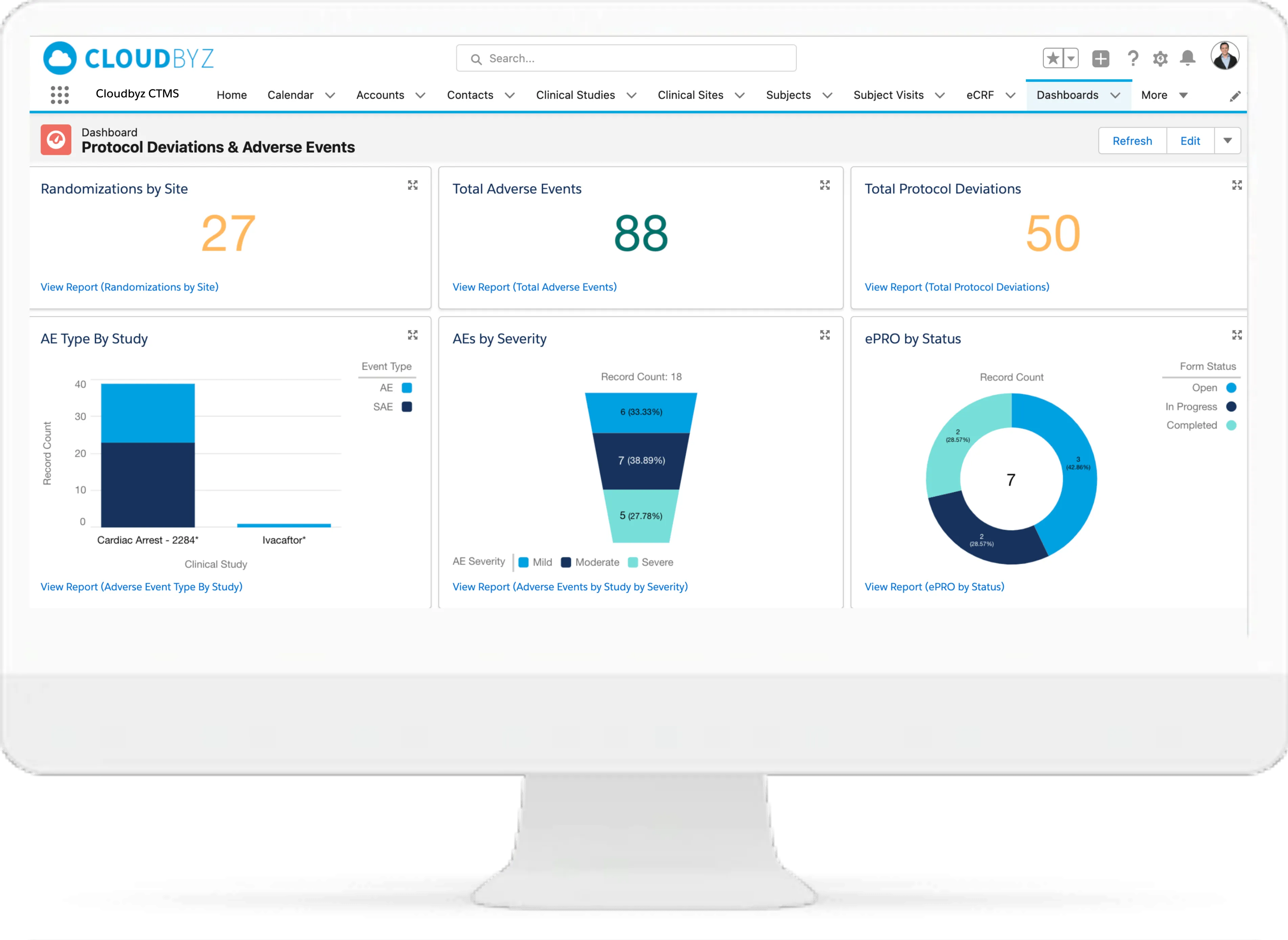
- Clinical Monitoring: Schedule and manage monitoring visits based on predefined plans.
- eTMF/eReg: Centralized document repository with regulatory tracking and compliance support.
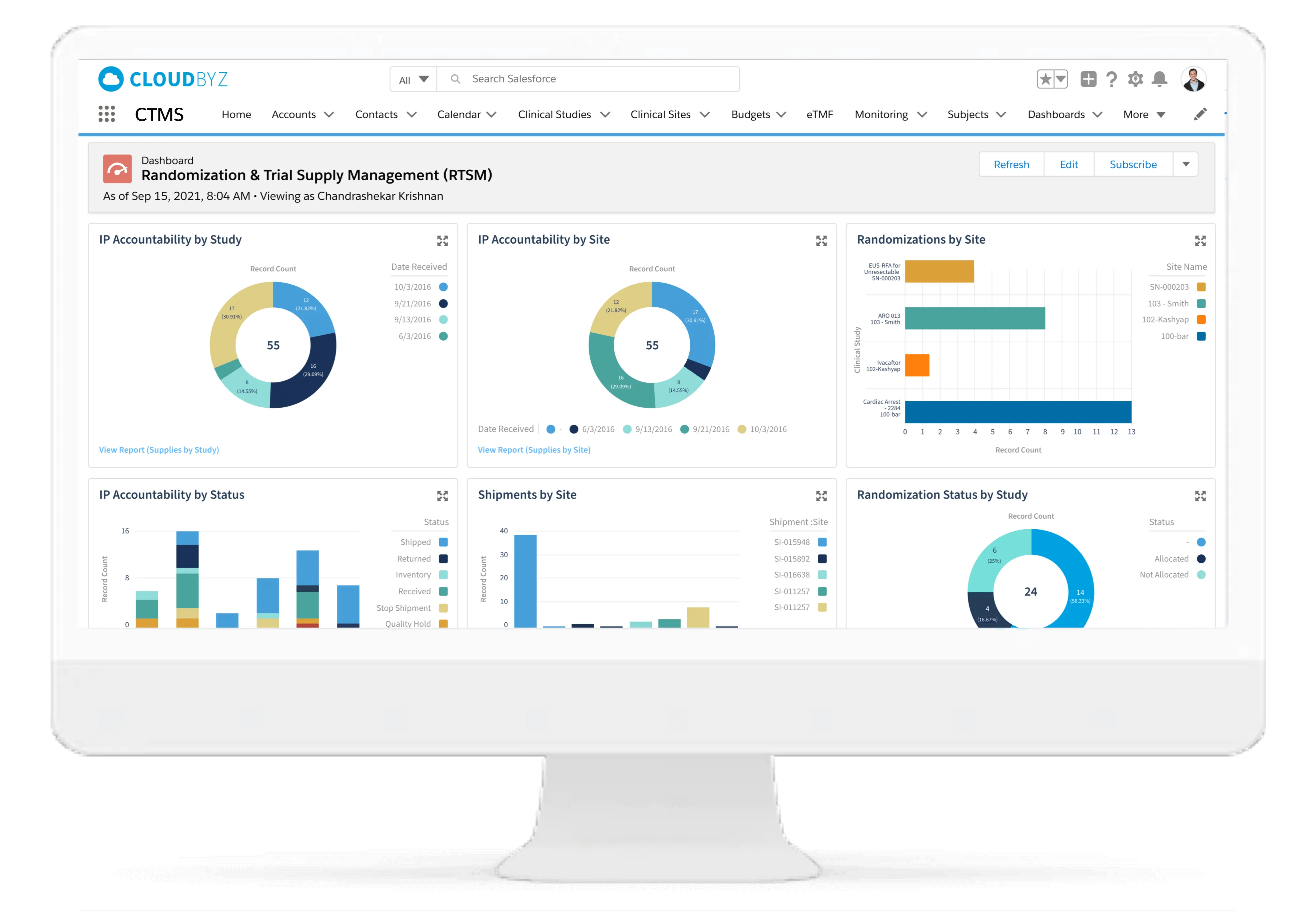
- Randomization & Trial Supply Management: Manage drug supply logistics and randomization schemes.
- Budget Management: Define and track study and site budgets with templates and real-time analytics.
- Payments: Automate payment workflows including advance, milestone, and holdback payments.
- Patient Recruitment: Manage recruitment campaigns, pre-screening, and communication workflows.
Capabilities
- End-to-End Trial Oversight: Supports full lifecycle management from planning to close-out.
- Real-Time Data Access: Centralized dashboards and analytics for informed decision-making.
- Integrated Collaboration: Facilitates communication between sponsors, CROs, and sites.
- Regulatory Compliance: Built-in support for FDA 21 CFR Part 11, HIPAA, and global standards.
- Customizable Workflows: Configurable modules to adapt to specific organizational needs.
- Salesforce Native: Leverages Salesforce’s scalability, security, and low-code flexibility.
- Audit Trails and E-Signatures: Ensures traceability and compliance with regulatory requirements.
Benefits
- Accelerates trial setup and execution timelines.
- Improves operational efficiency and reduces manual effort.
- Enhances data accuracy and quality across trial phases.
- Enables proactive risk management and compliance tracking.
- Boosts collaboration and transparency among stakeholders.
- Reduces costs through automation and centralized control.
- Scales easily to support global, multi-phase studies.